How to Calculate Specific Heat Capacity
Poundshour X specific heat X ΔT BTUs per hour. Water - Specific Heat vs.

Specific Heat Capacity Problems Calculations Chemistry Tutorial Ca Learn Physics Chemistry Jokes Chemistry
When two objects at the same mass are heated at equal.

. Specific heat capacity c of a substance is the heat capacity of an illustration of the substance divided by the mass of the model. Multiply to calculate BTUs per hour. At ambient pressure and temperature the isobaric.
What is the final temperature of the ethanol. Place the immersion heater into the central hole at the top of the calorimeter. Heat capacity is an extensive propertyThe corresponding intensive property is the specific heat capacity found by dividing the heat capacity of an object.
Specific Heat capacity of some substances at Atmospheric Temperature 20C. When heat transfer is involved use this formula. Water has a specific heat of c 4186Jg o C.
For the same battery a discharge current of. Please note that in Heat capacity we consider the specific amount of mass and that mass can be any amount. For example a constant discharge current of 1 C 5 A can be drawn from a 5 Ah battery for 1 hour.
Where Q is amount of heat T specific refers to temperature. A high heat capacity means that a substance can absorb a lot of heat before registering a change in temperaturethink about how long it takes for a pot to get warm to the touch on the stove versus how long it takes the water inside to get warm. Know that specific heat refers to the energy needed to raise one gram by one degree.
Battery capacity is defined as the total amount of electricity generated due to electrochemical reactions in the battery and is expressed in ampere hours. Water absorbs heat but without a sudden rise in its. Specific Heat capacity in J kg 1 K 1.
BTUs per hour 12000 Tons per hour. Heat Capacity is described in Joule per Kelvin JK. The heat capacity problem can be applied to calculate the heat capacity mass or temperature difference of any given substance.
The amount of heat that is required to raise the temperature of a gram of a substance by 1 degree Celsius is known as specific heat capacity. Specific heat C is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Change in temperature Q cm to calculate the change in temperature from a specific amount of heat added.
Formula for Heat Capacity. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. Tons per hour x 12 safety factor Chiller size in tons.
The formula of Specific Heat Capacity. Temperature - Online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 C 32-700 F - SI and Imperial units. Specific heat is a measure of heat capacity or how much heat a material can store when changing temperature.
The specific heat of ethanol is 244 JgC. Add a safety factor of 10 to 20. Convert BTUs to tons.
Q represents the heat added c is the specific heat capacity of the substance youre heating and m is the mass of the substance youre heating. Specific heat tells you the amount of energy needed to raise each unit one degree. Units of Heat - BTU Calorie and Joule - The most common units of heat BTU - British Thermal Unit Calorie and Joule.
Heat energy QT. Ahmet Aktaş Yağmur Kirçiçek in Solar Hybrid Systems 2021. The SI unit of heat capacity is joule per kelvin JK.
This is how specific heat capacity can be used to calculate the. Isobaric specific heat C p is used for ethanol in a constant pressure ΔP 0 system. This method is ideal for measuring the actual load of a process.
This example problem demonstrates how to calculate the final temperature of a substance when given the amount of energy used the mass and initial temperature. For example it takes 417 Joules to raise. When you find the heat capacity of one unit of something 1 gram 1 ounce 1 kilogram etc youve found this objects specific heat.
Calculate a simplified MCΔT. Specific heat is also now and again insinuated as massic heat. Isochoric specific heat C v is used for ethanol in a constant-volume isovolumetric or isometric closed system.
To measure the specific heat capacity of water. 300 grams of ethanol at 10 C is heated with 14640 Joules of energy. CQmΔT The unit of Specific heat capacity is.
Place one litre 1 kg of water in the calorimeter.

Specific Heat Capacity Explained Youtube Business Plan Template Word Chemistry Lessons Letter Reversal Worksheets

Specific Heat Capacity Explained Youtube Gcse Physics Gcse Science School Help

Specific Heat Capacity Amount Of Energy Needed To Change Temperature Of An Object By One Degr Business Plan Template Word Chemistry Lessons Teaching Chemistry
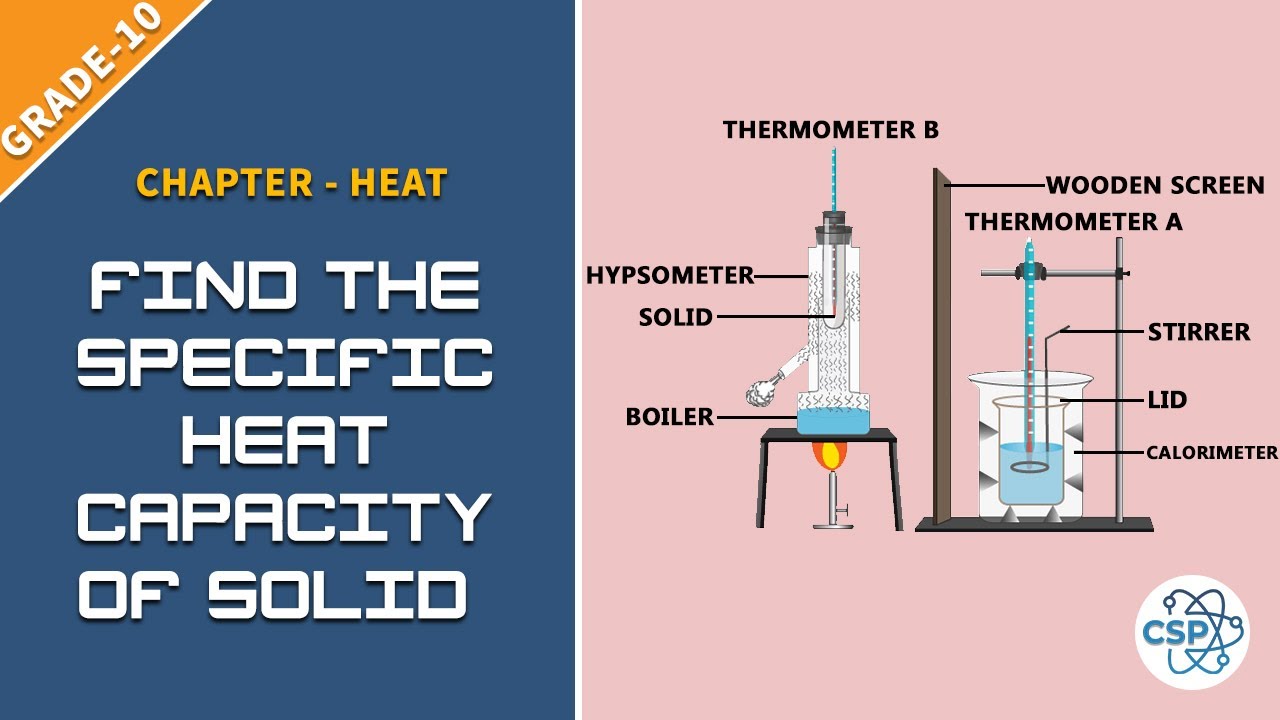
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments
0 Response to "How to Calculate Specific Heat Capacity"
Post a Comment